HARD
Earn 100
Zinc is more reactive than?
(a)Copper
(b)Iron
(c)Magnesium
(d) copper and iron.
50% studentsanswered this correctly
Important Questions on Sorting Materials into Groups
EASY
EASY
EASY
EASY
MEDIUM
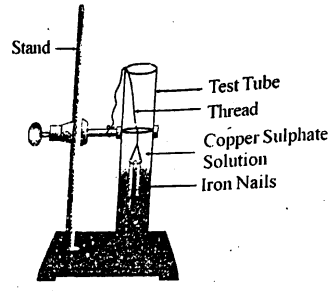
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
MEDIUM
EASY
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
Complete the following reaction:
EASY
EASY
EASY
HARD
MEDIUM

