EASY
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Zinc metal is extracted from its oxide. In the industrial extraction process, 5 tonnes of zinc oxide are needed to produce 4 tonnes of zinc. Calculate the mass of zinc, in tonnes, that is produced from 20 tonnes of zinc oxide.
50% studentsanswered this correctly

Important Questions on Quantitative Chemistry
EASY
Upper Secondary-IGCSE
IMPORTANT
Nitrogen and hydrogen react together to form ammonia.
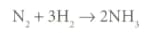
When the reaction is complete, 14 tonnes of nitrogen are converted into 17 tonnes of ammonia. How much nitrogen will be needed to produce 34 tonnes of ammonia?
EASY
Upper Secondary-IGCSE
IMPORTANT
EASY
Upper Secondary-IGCSE
IMPORTANT
• 2 atoms of carbon (C)
• 2 atoms of oxygen (O)
• 4 atoms of hydrogen (H).
What is the formula of a molecule of Y?
