MEDIUM
AS and A Level
IMPORTANT
Earn 100
Zirconium, , and hafnium, , are metals.
The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. A beam of protons is fired into an electric field produced by two charged plates, as shown in the diagram.
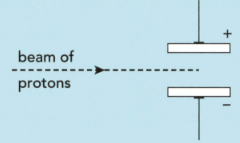
i) Describe how the beam of protons behaves when it passes through the gap between the charged plates.
ii) Explain your answer.

i) Describe how the beam of protons behaves when it passes through the gap between the charged plates.
ii) Explain your answer.

Important Questions on Atomic Structure
EASY
AS and A Level
IMPORTANT
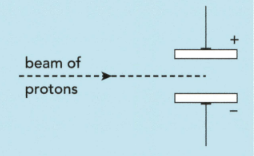
The subatomic particles present in zirconium and hafnium are electrons, neutrons and protons. Describe and explain what happens when a beam of neutrons passes through the gap between the charged plates instead of protons in the figure:
HARD
AS and A Level
IMPORTANT
Describe the structure of an atom, giving details of the subatomic particles present.
MEDIUM
AS and A Level
IMPORTANT
Explain the terms atomic number and nucleon number.
MEDIUM
AS and A Level
IMPORTANT
Complete the table:
| Neutral atom | Atomic number | Nucleon number | Number of each sub-atomic particle present |
| 12 | 24 | ||
| 13 | 27 |
EASY
AS and A Level
IMPORTANT
Explain why atoms are neutral.
EASY
AS and A Level
IMPORTANT
An oxygen atom has 8 protons in its nucleus. Explain why it cannot have 9 protons.
MEDIUM
AS and A Level
IMPORTANT
When deducing the relative mass of an atom, the electrons are not used in the calculation. Explain why not.
EASY
AS and A Level
IMPORTANT
The table below shows the two naturally occurring isotopes of chlorine. Complete the table:
| number of protons | ||
| number of electrons | ||
| number of neutrons |
