HARD
10th CBSE
IMPORTANT
Earn 100
is measured on a scale of to , with lower values indicating high hydrogen ion concentration (more acidic) and higher values indicating low hydrogen ion concentration (less acidic). A of is considered as neutral. Every whole unit in represents a ten-fold increase in or decrease in hydrogen ion concentration. What would the hydrogen ion concentration of a solution ofbe compared to a solution of ?

Important Questions on Acids, Bases and Salts
HARD
10th CBSE
IMPORTANT
Dipti has three flasks containing dilute hydrochloric acid, dilute sulphuric acid and dilute sodium hydroxide respectively. The flasks are not labelled, and she does not have any pH indicator.
(a) Which of the solutions will she be able to identify just by making mixtures of pairs of the substances.
(b) What observation will help her to make this identification?
HARD
10th CBSE
IMPORTANT
Which one of the following is the correct method of finding the of a solution?
HARD
10th CBSE
IMPORTANT
Bottle A contains acetic acid and bottle B contains sodium carbonate solution. When pH paper is dipped in each of the solution, the colour seen in A and B respectively will be:
HARD
10th CBSE
IMPORTANT
In slaking of lime, lime reacts vigorously with water to form lime water with releasing heat. Which statement(s) is/are true about the reaction?
(i) it is an endothermic reaction
(ii) it is an exothermic reaction
(iii) the pH of resulting solution is <
(iv) the pH of resulting solution is >
HARD
10th CBSE
IMPORTANT
A student was given three solutions marked X, Y and Z, and asked to arrange them in the increasing order of their pH values. The student put two drops of each solution on three strips of universal indicator paper separately. The colours shown by the three indicator strips are as follows:
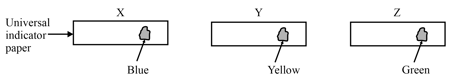
Which of the following gives the correct order of increasing pH values.
HARD
10th CBSE
IMPORTANT
A student added blue litmus solution to a colourless aqueous solution. The solution turned red. Which one of the following chemicals should be added in excess so that the change in colour is reversed?
HARD
10th CBSE
IMPORTANT
A student prepared hydrogen chloride gas by treating sodium chloride with concentrated sulphuric acid in test-tube. He held a strip of dry blue litmus paper in gas coming out of the test-tube. The student observed that on coming in contact with gas, the colour of blue litmus paper:
HARD
10th CBSE
IMPORTANT
A student added a drop of universal indicator to 1 ml of the given solution and found that a green colour is produced. pH value of the solution will be in the range of :
