EASY
Earn 100
what is a titrant?
Important Questions on Atoms, Molecules and Stoichiometry
EASY
EASY
HARD
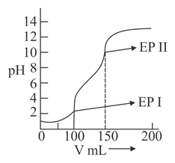
A weak acid (or base) is titrated against a strong base (or acid) and the volume V of a strong base (or acid) is plotted against the of the solution (as shown in figure). The weak electrolyte (i.e. acid or base) could be:
HARD
HARD
EASY
MEDIUM
MEDIUM
Which of the following solutions have the same concentration?
HARD
HARD
EASY
EASY
MEDIUM
The volume of required to dissolve of copper carbonate is:
MEDIUM
MEDIUM
MEDIUM
MEDIUM

