Atomic Radii, Ionic Radii and Vanderwall Radii
Atomic Radii, Ionic Radii and Vanderwall Radii: Overview
This topic covers concepts, such as Screening Effect, Effective Nuclear Charge, Calculation of Effective Nuclear Charge, Applications of Effective Nuclear Charge, Atomic Radius, Covalent Radius, Metallic Radius, Van der Waals Radius, etc.
Important Questions on Atomic Radii, Ionic Radii and Vanderwall Radii
The ions are isoelectronic. Their ionic radii show:
In which of the following arrangements the order is not according to the property indicated against it ?
The correct order of ionic radius is:
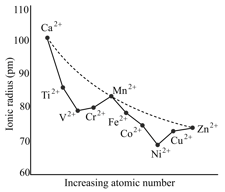
Metal ions in the fourth period are expected to show decrease in their ionic radii of the ions from to , due to the increase in the nuclear charge, as shown by the dotted line in the Figure given below. When they form octahedral complexes with weak field ligands, the expected regular decrease is not observed, as shown by the solid line in the Figure. The correct statement(s) is/are
Select the incorrect orders about the size of the given species:
Assertion. and are isoelectronic but the magnitude of the ionic radius of is less than that of .
Reason. The magnitude of effective nuclear charge of the outer shell electrons in is greater than in .
The atomic radii of and follow the order:
Identify the correct order of ionic radii of the given species:
Which among the following iso-electronic species has the smallest size?
The ions are iso-electronic. Their ionic radii show
Which of the following has highest value of ionic radius?
The correct order of radius for the following elements is
The ionic radii of follow the order
The correct order of energy of -orbitals in , , and , is
Nuclear charge increases with an increase in ________.
In crystals of which of the following ionic compounds, would you expect the maximum distance between centres of cations and anions.
Which among the following species has the same number of electrons in its outermost as well as penultimate shell ?
The correct arrangement for the ions in the increasing order of their radii is
The correct order of decreasing ionic radii among the following isoelectronic species is
For the correct order of increasing ionic radii is
