Basic Character of Amines
Basic Character of Amines: Overview
This topic describes the basic character of amines with the reason for its basicity. It also mentions the groups that can increase or decrease the basic character of amines. We will learn about the order of basicity in amines.
Important Questions on Basic Character of Amines
The increasing order of basic strengths in their aqueous solutions is
State which of the following statements are true:
(i) value for aniline is less than that for methylamine.
(ii) Methylamine in water reacts with ferric chloride to give a precipitate of ferric hydroxide.
(iii) Aniline does not undergo Friedel-Crafts reaction.
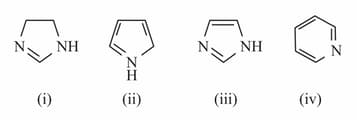
The order of basic strength in gas phase of the following compound would be:
The order of basic strength in gas phase of the following compound would be:
(i) Aniline does not undergo Friedel-Crafts Reaction.
(ii) Aliphatic amines are stronger bases than aromatic amines.
State if the above two statements are true or false:
Which one of the following is the strongest base in aqueous solution?
Amongst the following the most basic compound is –
Amongst the following the most basic compound is:
The correct order of increasing basic nature for the bases and is –
The correct order of increasing basic nature for the bases and is:
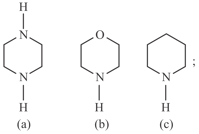
Compare basic strength of :
The correct decreasing order of the basic strength is
Arrange the following in decreasing order of their values
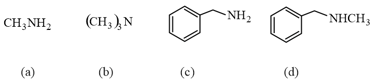
Among methanamine, ethanamine, benzenamine, -methylaniline and , -dimethylaniline, the weakest and the strongest base in aqueous phase, respectively are
Which is the correct basic strength order of the following compounds?
i)
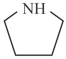
ii)
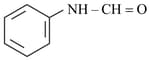
iii)
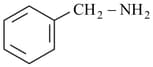
iv)
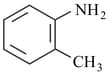
The decreasing order of basicity of following aniline derivatives is:
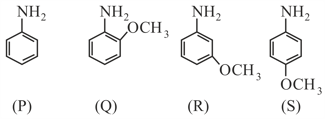
Which of the following compound has least Basic strength?
The CORRECT order of basic strength of following compounds is
What is the increasing order of basic strength of the following compounds in aqueous solution?
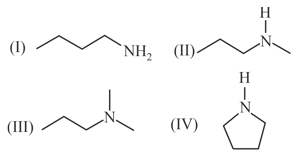
Arrange the following compounds in order of decreasing basicity.