Adsorption
Adsorption: Overview
This Topic covers sub-topics such as Absorption, Adsorption, Adsorption Isotherms, Freundlich's Adsorption Isotherm, Adsorbent, Desorption, Physical Adsorption, Adsorbate, Sorption, Mechanism of Adsorption and, Chemical Adsorption
Important Questions on Adsorption
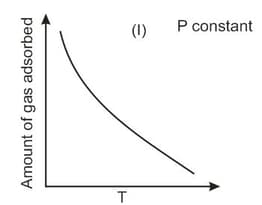
The given graphs/data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice(s) about I, II, III and IV is (are) correct?
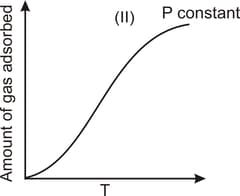
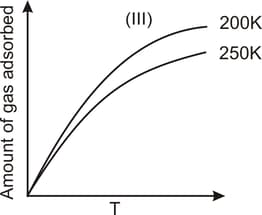
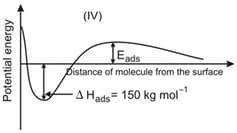
Among physisorption and chemisorption, which type of a adsorption has a higher enthalpy of adsorption?
20% of surface sites are occupied by molecules. The density of surface sites is and total surface area is . The catalyst is heated to 300 K while is completely desorbed into a pressure of 0.001 atm and volume of . The number of active sites occupied by each molecule will be
Adsorption of gases on solid surface is generally exothermic because:
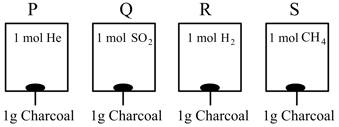
From the figure, in which of the following vessel, the pressure of the gas is the highest. [Temperature and volume of the gases are the same in each vessel].
What is the effect of surface area on physical adsorption?
There is no effect of surface area on physical adsorption.
There is an effect of surface area on physical adsorption.
How does surface area affects physical adsorption?
Which type of layer is formed in physical adsorption?
Name the adsorbents widely used in chromatographic analysis.
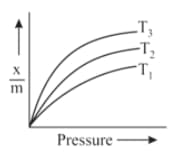
Which curve shows the maximum adsorption in the given graph.
Adsorption indicators are used to detect the end point in which type of reactions?
The rate of physical adsorption increases with
A mixture of noble gases can be separated by: