Electrolysis
Electrolysis: Overview
This topic covers concepts, such as Electrolysis, Electrolytic Cells & Products of Electrolysis etc.
Important Questions on Electrolysis
In the electrolytic cell, flow of electrons is from
A dilute aqueous solution of is electrolysed using platinum electrodes. The products at the anode and the cathode are
During the electrolysis of copper sulphate solution, the mass of anode gradually:
Copper sulphate solution (blue colour) is electrolysed using copper electrodes. The colour of the copper sulphate changes to:
The diagram shows the electrolysis of molten . What occurs when the switch is closed?
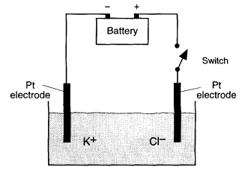
Which of the following is true for an electrolytic cell?
An iron ring is plated with zinc metal in a cell. Which of the following is true?
During electrolysis, the positive ions move towards the
Electrolysis of aqueous with inert electrodes gives
gives hydrogen with and but not with because
What will be products of electrolysis of an aqueous solution of with silver electrodes?
During electrolysis, the species discharged at cathode are:
Electrolysis involves oxidation and reduction respectively at:
Which of the following compounds will not undergo decomposition on passing electricity through the aqueous solution?
The amount of electricity required to produce mole of from solution will be
During the electrolysis of molten solution, of sodium metal is deposited at the cathode. How many moles of chlorine will be obtained at anode?
Aluminium is extracted from alumina (Al2O3) by electrolysis of a molten mixture of
