Characteristic Properties of Gases
Characteristic Properties of Gases: Overview
This Topic covers sub-topics such as Physical Properties of a Gas
Important Questions on Characteristic Properties of Gases
Which of the following pairs contain gases heavier than ?
of gas diffuses through a small hole in a container in time . How much time will be required by oxygen gas for the diffusion of same volume?
The rate of diffusion of a gas having molecular weight just double of hydrogen gas is . The rate of diffusion of hydrogen gas will be
Density of gaseous mixture and from percentage volume is given as
Pure diffuses through an aperture in where as mixture of and another gas containing diffuses from the same in What is molecular weight of the gas.
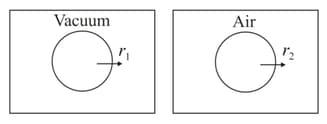
A gas is effusing in vacuum at rate and in air at rate , then :
Give one word for the following.
Movement of one gas molecule into another gas.
Which description fits best for a gas?
At constant pressure and temperature the rates of diffusion and of gases and having densities and are related as :-
A manometer reads the pressure of a gas in an enclosure as shown in the figure :
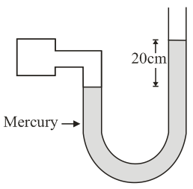
The absolute and gauge pressure of the gas in of mercury is (Take atmospheric pressure of mercury)
There are large vacant space between the molecules known as _____.
What are the measurable properties of gases?
A certain gas takes three times as long to effuse out as helium. Its molecular mass will be
List the characteristic physical properties of the gases.
Name the term used for mixing of different gases by random molecular motion and frequent collision. (diffusion/osmosis)
Diffusion of one mole of Nitrogen gas at 08 atm takes 38 seconds through a pinhole, whereas diffusion of one mole of an unknown compound of xeon with fluorine at 1.6 atm takes 57 seconds through the same hole. Find out the molecular formula of the compound.
When a balloon of radius having of mass is filled with helium, at bar in which given density of air is and bar , the pay load will be:
At gas is leaked through a tiny hole into a vessel for minutes. Another unknown gas, at the same temperature and pressure, as that of is leaked through the same hole for minutes. The mixture of gases exerts a pressure of after the effusion. If concentration of is and volume of vessel is litre, then calculate the molecular weight of the unknown gas.
“When stating the volume of gas, the pressure and temperature should also be given.” Why?
Cotyledons are also called-
