Colloidal State
Colloidal State: Overview
This topic discusses the colloidal state. It covers the theory of classification of solute as crystalloid and colloid on the basis of the size of the particle of solute.
Important Questions on Colloidal State
Substances whose solutions can pass through filter paper as well as animal membrane are called
Which of the following is not a colloid?
Give any two differences between Colloidal Solution, True Solution and Suspension.
Size of colloidal particle ranges between
In _____ (colloids/suspension), particles have a diameter greater than 1000 nm.
In suspension, particles have a diameter greater than 1000 nm.
Colloids are the type of mixture, where the solute (tiny particles) is uniformly distributed in the solvent. Particle size if between 1-1000 nm.
_____ (true solutions/colloids) are the type of mixture, where the solute (tiny particles) is uniformly distributed in the solvent. Particle size if between 1-1000 nm.
Classify solutions on the basis of particle size.
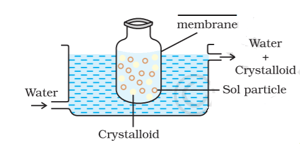
Complete the blank in the above diagram of dialysis.
Which of the following is a natural colloid ?
Give reasons.
Same compound can acts as both crystalloid and colloid.
Explain why colloidal particles in sols are good adsorbent.
Which one of the following is not a colloidal system: