Shikha Goel and Saleha Parvez Solutions for Chapter: Chemical Changes and Reactions, Exercise 5: CHALLENGERS*
Shikha Goel Chemistry Solutions for Exercise - Shikha Goel and Saleha Parvez Solutions for Chapter: Chemical Changes and Reactions, Exercise 5: CHALLENGERS*
Attempt the practice questions on Chapter 2: Chemical Changes and Reactions, Exercise 5: CHALLENGERS* with hints and solutions to strengthen your understanding. All In One ICSE Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Shikha Goel and Saleha Parvez Solutions for Chapter: Chemical Changes and Reactions, Exercise 5: CHALLENGERS* with Hints & Solutions
Which of the following colour change occur when the gases after thermal decomposition of ferrous sulphate came in contact with an acidified solution of potassium dichromate?
Consider the following equation for the process of photosynthesis, used by green plants to prepare their own food.
Choose the correct words for and .
A precipitation reaction is one in which two soluble reactants form an insoluble product. The insoluble product is a solid which usually sinks to the bottom of the liquid.
The table shows the solubility of the compounds that form when some chemicals react. The compounds that are soluble are marked with a tick (right). The compounds that are insoluble are marked with a cross(x).
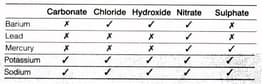
Which of the following word equations represents a precipitation reaction?
Which of the following is correct order of reactivity of element?
What happens when ferrous sulphate crystals are heated?
The carbonate of metal X is a white solid. It decomposes when heated to form carbon dioxide and a yellow solid oxide. What is metal X?
Sonu wanted to react potassium iodide solution with aqueous solution of lead nitrate to obtain a yellow precipitate of lead iodide. To carry out the reaction, he made the following experimental set up.
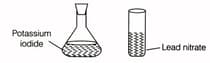
But accidently the lead nitrate solution fall down and more lead nitrate is not available in the laboratory. Could you suggest which of the following salt he can use for performing this activity?
When mercury (II) thiocyanate is heated
I. A decomposition reaction takes place.
II. An endothermic reaction takes place.
III. Carbon disulphide gas is evolved.
The true statements are
