Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 3: Exercise-3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 3: Exercise-3
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 3: Exercise-3 with Hints & Solutions
The rate constant for the reaction is If the rate is then the concentration of (in ) is:
In the biologically -catalysed oxidation of ethanol, the concentration of ethanol decreases in a first order reaction from to in The constant rate of the reaction is:
Plots showing the variation of the rate constant with temperature are given below. The plot that follows Arrhenius equation is:
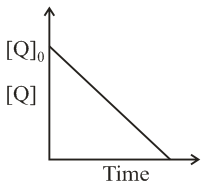
In the reaction,
the time taken for reaction of , is twice the time taken for reaction of The concentration of varies with reaction time as shown in the figure. The overall order of the reaction is:
For the reaction rate is given then the order of the reaction is:
The half life of a radio isotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 hours undecayed is
For the reaction, the differential rate law can be written as:
For an elementary chemical reaction,
the expressions for
