Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Exercise-3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Exercise-3
Attempt the free practice questions on Chapter 12: Surface Chemistry, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Surface Chemistry, Exercise 3: Exercise-3 with Hints & Solutions
The given graph / data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and pressure. Which of the following choice (s) about I, II, III and IV is (are) correct?
(i) 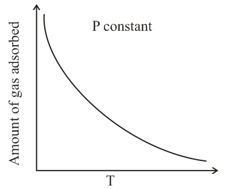 (ii)
(ii) 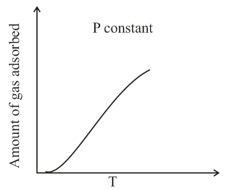 (iii)
(iii) 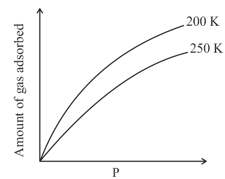
The qualitative sketches I, II and III given below show the variation of surface tension with mołar concentration of three different aqueous solution of and at room temperature. The correct assignment of the sketches is:
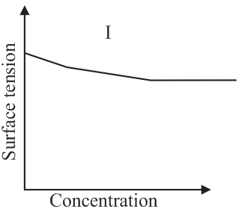
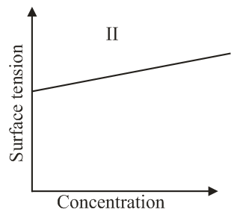
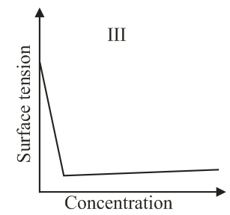
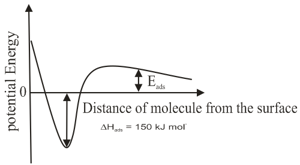
The correct statement(s) about surface properties is(are)
gram of activated charocoal was added to of acetic acid solution in a flask. After an hour it was filtered and the strength of the fitrate was found to be . The amount of acetic acid adsorbed (per gram of charcoal) is:
Under ambient conditions, which among the following surfactants will form micelles in aqueous solution at lowest molar concentration ?
Among the following, the correct statement is:
Adsorption of a gas follows Freundlich adsorption isotherm. In the given plot, is the mass of the gas adsorbed on mass of the adsorbent at pressure . is proportional to:
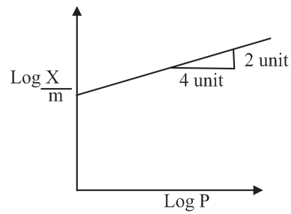
An example of solid sol is:
