Embibe Experts Solutions for Chapter: Solutions, Exercise 5: Exercise 5
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 5: Exercise 5
Attempt the practice questions on Chapter 8: Solutions, Exercise 5: Exercise 5 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 5: Exercise 5 with Hints & Solutions
The boiling points of two miscible liquids, which do not form an azeotropic mixture, are close to each other. Their separation is best carried out by:
When swimming for a long time in salt water, the skin of one's fingertips wrinkles. Which one of the following property is responsible for this observation?
According to this phase diagram, which phases can exist at pressures lower than the triple point pressure?
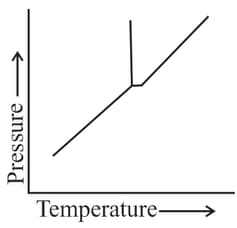
The aqueous solution having osmotic pressure nearest to that of an equimolar solution of is:
The elevation in the boiling point of a solution containing of in of water is
Which of the following observation indicates colligative property?
A solution has a higher vapour pressure than .
A solution freezes at a lower temperature than pure water.
Pure water freezes at a higher temperature than pure ethanol.
The colligative property used in the determination of the molar mass of a polymer is
Density of solution of a non-electrolyte is . If is , solution freezes at
