Embibe Experts Solutions for Chapter: Electrochemistry, Exercise 4: EXERCISE-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Electrochemistry, Exercise 4: EXERCISE-4
Attempt the free practice questions on Chapter 9: Electrochemistry, Exercise 4: EXERCISE-4 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Electrochemistry, Exercise 4: EXERCISE-4 with Hints & Solutions
For the galvanic cell.
Calculate the EMF generated and assign correct polarity to each electrode for a spontaneous process after taking into account the cell reaction at
(aq) was electrolyzed using inert electrodes by passing till the of the resulting solution was . The solution after electrolysis was neutralized, treated with excess and titrated with . Volume of required was . Assuming no volume change during electrolysis, calculate:
(a) duration of electrolysis if current efficiency is
(b) initial concentration of
Calculate the equilibrium concentration of all ions in an ideal solution prepared by mixing of with of .
Determine at for cell :
(a) it's emf when
(b) the when
(c) the positive electrode when
given
Carefully observe the given figure and using data provided find the EMF of shown Galvenic cell in volt :
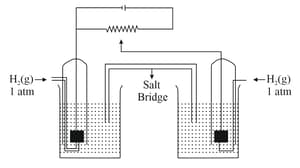
Solution is each in and and solution is each in and
[Given : and volt]
Dal lake has water litre approximately. A power reactor produces electricity at the rate of coulomb per second at an appropriate voltage. How many years would it take to electrolyse the lake?
Calculate the cell potential of a cell having reaction in a solution buffered at and which is also saturated with .
For and .
Calculate the solubility and solubility product of in water at from the following data : Conductivity of a saturated solution of is and that of water used . The ionic molar conductivities of and are and
