Embibe Experts Solutions for Chapter: Solutions, Exercise 2: EXERCISE-2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 2: EXERCISE-2
Attempt the free practice questions on Chapter 8: Solutions, Exercise 2: EXERCISE-2 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 2: EXERCISE-2 with Hints & Solutions
For a dilute solution containing a nonvolatile solute, the molar mass of solute evaluated from the elevation of boiling point is given by the expression:
For a dilute solution containing a nonvolatile solute, the molar mass of solute evaluated from the osmotic pressure measurement is given as:
An aqueous solution of acetone, , is acetone by weight. What is the mole percentage of acetone in this solution:
of is partial pressure of is mole fraction of in liquid phase in the mixture of two liquids and and is the mole fraction of in vapour phase, then in of is:
If the vapour pressure of a pure solvent and $\mathrm{P}$ is the vapour pressure of the solution prepared by dissolving a non volatile solute in it. The mole fraction of the solvent is given by:
The following graph represents variation of boiling point with composition of liquid and vapours of binary liquid mixture. The graph is plotted at constant pressure, for a solution of mole fraction
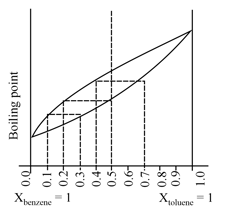
Which of the following statement(s) is correct.
Which of the following plots represents an ideal binary mixture?
Acetone and carbon disulphide form binary liquid solution showing positive deviation from Raoult law. The normal boiling point of pure acetone is less than that of pure . Pick out the incorrect statements among the following.
