Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3
Attempt the free practice questions on Chapter 18: Kinetic Theory of Gases, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Kinetic Theory of Gases, Exercise 3: Exercise-3 with Hints & Solutions
An ideal gas having initial pressure , volume and temperature is allowed to expand adiabatically until is volume becomes while its temperature falls to .
(i) How many degrees of freedom do gas molecules have?
(ii) Obtain the work done by the gas expansion as a function of the initial pressure and volume . [Take
An ideal gas has a specific heat at constant pressure . The gas is kept in a closed vessel of volume , at a temperature of and a pressure of . An amount of of heat energy is supplied to the gas. If the final temperature and pressure of the gas are and respectively then find .
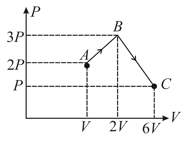
One mole of monoatomic ideal gas undergoes a process as shown in figure. The maximum temperature of the gas during the process is in the form Find .
An ideal monoatomic gas occupies volume at temperature and pressure . The internal energy of the gas is taken to be zero at this point. It undergoes the following cycle : The temperature is raised to at constant volume, the gas is then expanded adiabatically till the temperature is , followed by isothermal compression to the original volume. Calculate (i) The work done and the heat transferred in each process and the internal energy at the end of each process, (ii) The thermal efficiency of the cycle.
A vertical cylinder of cross-sectional area closed at both ends is fitted with a frictionless piston of mass dividing the cylinder into two parts. Each part contains one mole of an ideal gas in equilibrium at . The volume of the upper part is and that of the lower part is . What force must be applied to the piston so that the volumes of the two parts remain unchanged when the temperature is increased to ?
There is a soap bubble of radius in air cylinder which is original at the pressure of . The air in the cylinder is now compressed isothermally until the radius of the bubble is halved. Now the pressure of air in the cylinder. becomes . Find the value of . (The surface tension of the soap film is ).
There is soap bubble of radius in air cylinder which is originally at the pressure of The air in the cylinder is now compressed isothermally until the radius of the bubble is halved. Calculate now the pressure (in ) of air in the cylinder. The surface tension of the soap film is
An air bubble doubles its volume as it rises from the bottom of a tank to its surface. If the atmospheric pressure be of what is the depth (in metre) of the tank?
