Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 5: Exercise 3.5
Richard Harwood Chemistry Solutions for Exercise - Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 5: Exercise 3.5
Attempt the free practice questions on Chapter 3: Elements and Compounds, Exercise 5: Exercise 3.5 with hints and solutions to strengthen your understanding. Cambridge IGCSE Chemistry Workbook 4th Edition solutions are prepared by Experienced Embibe Experts.
Questions from Richard Harwood and Ian Lodge Solutions for Chapter: Elements and Compounds, Exercise 5: Exercise 3.5 with Hints & Solutions
The diagram below shows a representation of the structure of an ionic oxide.
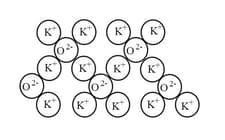
What is the ratio of K+ ions to O2-ions? _____ .
The diagram below shows a representation of the structure of an ionic oxide.
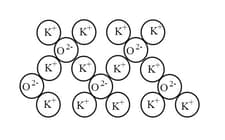
What is the formula of this compound?
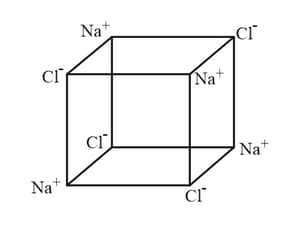
The following diagram shows the structure of common salt.
Extend the structure to the right, by adding four more ions.
Complete the diagrams below for the ions in the structure to show their electron arrangement. Draw in any missing electron shells, showing clearly the origin of the electrons involved.
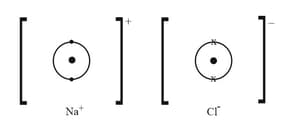
Draw an ionic diagram similar to the one above for the structure of magnesium chloride.
