Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise
Attempt the practice questions on Chapter 3: Chemical Kinetics, Exercise 1: Exercise with hints and solutions to strengthen your understanding. Chemistry Crash Course (Based on Revised Syllabus-2023) solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: Exercise with Hints & Solutions
In an elementary reaction: the molecularity of the reaction is _____.
of a first order reaction was completed in minutes. of the reaction will be completed in.
The half-life of a radioactive element is years. How long will it take to reduce to of its original mass?
Analyse the given graph, drawn between the concentration of reactant vs. time.
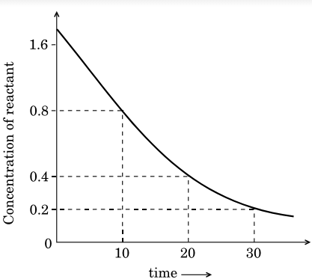
Predict the order of the reaction.
The temperature coefficient of most of the reactions lies between:
In the reaction the rate of appearance of bromine is related to the rate of disappearance of bromide ions as
For the reaction the value of rate of disappearance of is given as . The rate of formation of and is given respectively as :
The decomposition of in , at is studied by monitoring the concentration of in the solution. Initially the concentration of is and after minutes, it is reduced to . What is the rate of production of during this period in ?
