Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 7: Equilibrium, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Equilibrium, Exercise 1: Exercise 1 with Hints & Solutions
A buffer solution is in and in . If the same volume of another buffer is in and in is taken. Then, difference masses of could be added to these buffers before the of both buffers rise just above ? ( of )
A particular water sample has . What fraction of the water must be evaporated from the sample before solid begins to deposit of
There are stretchable balloons are filled up their maximum capacity. When an inert is introduced in these balloons at constant pressure. Which of the balloon will not burst?
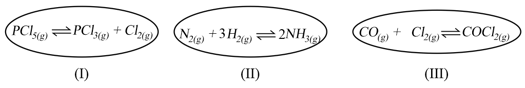
A certain gas '' polymerizes to a very small extent at a given temperature as . The reaction is started with one mole of '' in a container of capacity V. Which of the following is the correct value of , at equilibrium?
Consider the addition of and in water as given in (a) and (b)
(a) of in of pure water of
(b) $3.9 \mathrm{mg}$ of $\mathrm{CaF}_{2}$ in $100 \mathrm{~mL}$ of pure water, of . Choose the correct statement(s) from amongst the following.
A impure sample containing weak monoacidic base (Molecular weight ) is dissolved in water and titrated with at . When of the base is neutralized, the is found to be and at equivalent point, the of solution is ( antilog ).
Consider the equilibria:
1.
2.
(Haemoglobin in blood)
Identify the correct statement(s):
At , the progress of the reaction:
in a vessel is presented in the following figure:
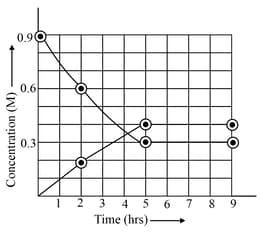
Identify the correct statement(s):
