Embibe Experts Solutions for Chapter: Solutions, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 14: Solutions, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 1: Exercise 1 with Hints & Solutions
An aqueous solution of percent (wt./wt.) non-volatile non-electrolyte solute, exerts a pressure of bar at the normal boiling point of solvent.
The molecular mass of solute is: ( bar)
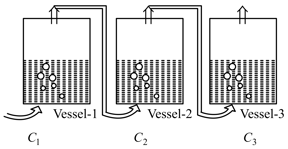
Dry air is slowly passed through three solutions of different concentrations, and ; each containing (non volatile) as solute and water as solvent, as shown in the figure. If the vessel gains weight and the vessel loses weight, then correct statement is
moles of is heated to form and . As soon as and are formed they react to form .
are simultaneously established. At equilibrium, the degree of dissociation of was found to be . Which of the following is/are incorrect at equilibrium?
Benzene and naphthalene form an ideal solution at room temperature. For this process, the true statement(s) is(are)
Identify the correct statements when a little amount of is added to solution
Which of the following changes decrease the vapour pressure of water
A closed jar having water vapours in equilibrium with liquid suddenly all the vapours of jar is transferred to another identical jar and is subjected to compression at same temperature and negligible volume occupied by the liquid water. Select the observation in the second jar.
Given:
Methylene blue, , Gelatin, Starch, Congo red, Number of common positive sols
Number of common negative sols , then value is
