Embibe Experts Solutions for Chapter: States of Matter: Gases and Liquids, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: States of Matter: Gases and Liquids, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 5: States of Matter: Gases and Liquids, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: States of Matter: Gases and Liquids, Exercise 1: Exercise 1 with Hints & Solutions
One mole of is taken in litre empty container fitted with a movable piston at If it is heated to at constant pressure then match the change (List) in parameters (List ) of gas as compared to initial state select the correct code.
| List (Parameter) | List (Change) | ||
| (number of collision made by a molecule per unit time) | |||
| (collision frequency) | |||
| (mean free path) | |||
| (root mean square speed) | |||
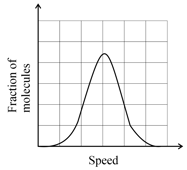
If the distribution of molecular speeds of a gas is as per the figure shown below, then the ratio of the most probable, the average, and the root mean square speeds, respectively, is:
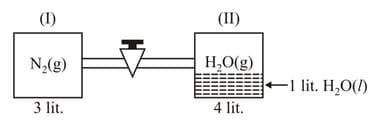
Two containers are connected by a tube of negligible volume, container has gas at pressure atm and temperature and container has litre at temperature initially. Find correct option(s) after stopcock is removed. (aq. Tension torr)
The correct statement regarding various types of molecular speeds are
Which of the following is/are correct according to kinetic theory of ideal gases:
A gas described by vander Waals equation
Energies associated with intermolecular forces.
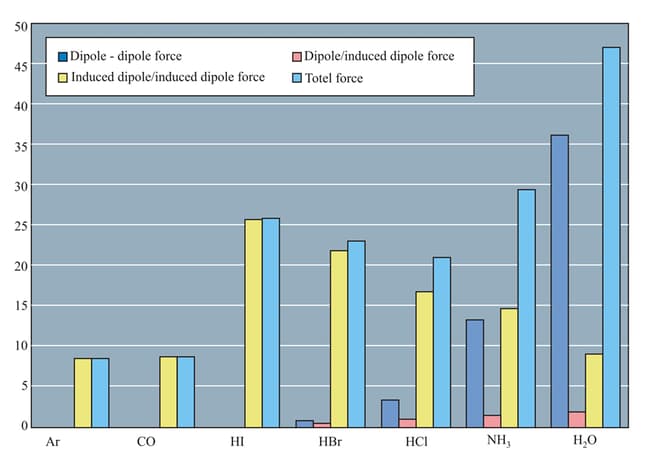
Which of the following statements is/are correct regarding intermolecular interactions:
Which of them is stabilised by intramolecular hydrogen bonding.
