Lawrie Ryan and Roger Norris Solutions for Chapter: Entropy and Gibbs Free Energy, Exercise 3: Question
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Entropy and Gibbs Free Energy, Exercise 3: Question
Attempt the free practice questions on Chapter 23: Entropy and Gibbs Free Energy, Exercise 3: Question with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Entropy and Gibbs Free Energy, Exercise 3: Question with Hints & Solutions
Refer to Figure below. If there are four molecules in the gas jar on the left, how many ways of arranging the molecules are there when the partition is removed?
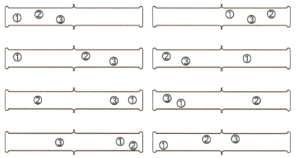
What is the probability of finding all four molecules in the right-hand gas jar as shown below the example?
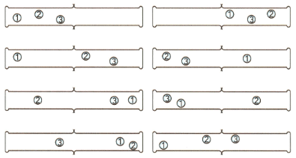
The following change is likely to be spontaneous ?
Sugar dissolving in water.
The following change is likely to be spontaneous?
The smell from an open bottle of aqueous ammonia diffusing throughout a room.
The following change is likely to be spontaneous?
Water turning to ice at .
The following change is likely to be spontaneous?
Ethanol vaporising at .
The following change is likely to be spontaneous or not?
Water mixing completely with cooking oil.
The following change is likely to be spontaneous or not?
Limestone(calcium carbonate) decomposing at room temperature.
