Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET 2019 (21-Apr Shift-2)
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET 2019 (21-Apr Shift-2)
Attempt the free practice questions on Chapter 27: Thermodynamics, Exercise 1: AP EAPCET 2019 (21-Apr Shift-2) with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: AP EAPCET 2019 (21-Apr Shift-2) with Hints & Solutions
A system is taken from state to state along two different paths. The heat absorbed and work done by the system along these two paths are and respectively, then
A gas is suddenly compressed to its initial volume. Then find the ratio of its final to initial pressure.
Isothermal process has non-linear graph between:
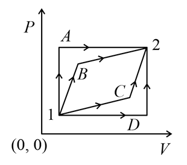
An ideal gas is taken from state- to state- through optional path and as shown in the diagram. Let and represent the heat supplied, work done and change internal energy respectively, then
Which one of the graphs below best illustrates the relationship between internal energy of an ideal gas and temperature of the gas in ?
For a monoatomic ideal gas following the cyclic process shown in the vs plot, identify the incorrect option:

A refrigerator with coefficient of performance releases of heat to a hot reservoir. The work done on the working substance is
A Carnot engine whose heat sink is at has an efficieny of . By how much should its source temperature be changed so as to increase its efficiency to ?
