Dr. J M D'Souza and Dr. Kirti J D'Souza Solutions for Chapter: Chemical Effects of Electric Current, Exercise 5: HIGHER ORDER THINKING SKILLS
Dr. J M D'Souza Science Solutions for Exercise - Dr. J M D'Souza and Dr. Kirti J D'Souza Solutions for Chapter: Chemical Effects of Electric Current, Exercise 5: HIGHER ORDER THINKING SKILLS
Attempt the free practice questions on Chapter 14: Chemical Effects of Electric Current, Exercise 5: HIGHER ORDER THINKING SKILLS with hints and solutions to strengthen your understanding. EUREKA PLUS Revised Bringing Science to Life solutions are prepared by Experienced Embibe Experts.
Questions from Dr. J M D'Souza and Dr. Kirti J D'Souza Solutions for Chapter: Chemical Effects of Electric Current, Exercise 5: HIGHER ORDER THINKING SKILLS with Hints & Solutions
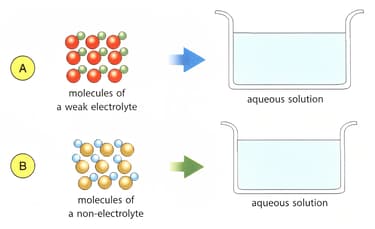
Draw atoms or molecules in containers A and B to show weak and non-electrolyte solutions.
In an electroplating chamber, the anode is a copper rod. What will happen to the anode if the chamber is used to coat copper on iron objects using copper sulphate solution? Will the electrode be damaged? Why?
A mixture of table salt and glucose powder is dissolved in distilled water. Will the solution conduct electricity? Why?
Give reason:
All compounds that dissolve in water are not electrolytes.
Give reason:
A bulb glows dimly when current is passed through a solution of vinegar.
Give reason:
Distilled water is not a conductor of electricity.
Give reason:
Bubbles form at the anode when current is passed through .
Give reason:
At times while electroplating a plate, a part of it is covered with wax.
