Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2018
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2018
Attempt the free practice questions on Chapter 13: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Advanced Paper 1 - 2018 with Hints & Solutions
A spherical bubble inside water has radius . Take the pressure inside the bubble and the water pressure to be The bubble now gets compressed radially in an adiabatic manner so that its radius becomes . For the magnitude of the work done in the process is given by where is a constant and . The value of is______
A mixture of ideal gas containing moles of monatomic gas and mole of rigid diatomic gas is initially at pressure volume and temperature If the gas mixture is adiabatically compressed to a volume , then the correct statement(s) is/are,
(Given is gas constant)
Answer the following by appropriately matching the lists based on the information given in the paragraph.
In a thermodynamics process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by where is temperature of the system and is the infinitesimal change in a thermodynamic quantity of the system. For a mole of monatomic ideal gas Here, is gas constant, is volume of gas, and are constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| List I | List II | ||
|---|---|---|---|
| (I) | Work done by the system in process | (P) | |
| (II) | Change in internal energy in process | (Q) | |
| (III) | Heat absorbed by the system in process | (R) | |
| (IV) | Heat absorbed by the system in process | (S) | |
| (T) | |||
| (U) |
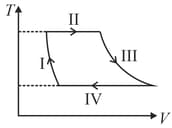
If the process carried out on one mole of monatomic ideal gas is as shown in figure in the - -diagram with the correct match is,
Answer the following by appropriately matching the lists based on the information given in the paragraph.
In a thermodynamics process on an ideal monatomic gas, the infinitesimal heat absorbed by the gas is given by where is temperature of the system and is the infinitesimal change in a thermodynamic quantity of the system. For a mole of monatomic ideal gas Here, is gas constant, is volume of gas, and are constants.
The List-I below gives some quantities involved in a process and List-II gives some possible values of these quantities.
| Column - I | Column - II | ||
| (I) | Work done by the system in process | (P) | |
| (II) | Change in internal energy in process | (Q) | |
| (III) | Heat absorbed by the system in process | (R) | |
| (IV) | Heat absorbed by the system in process | (S) | |
| (T) | |||
| (U) |
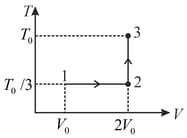
If the process on one mole of monatomic ideal gas is as shown in the -diagram with the correct match is
One mole of an ideal monatomic gas undergoes an adiabatic expansion in which its volume becomes eight times its initial value. If the initial temperature of the gas is and the universal gas constant , then how much is the decrease in its internal energy (in ) ?
Consider one mole of helium gas enclosed in a container at initial pressure and volume . It expands isothermally to volume . After this, the gas expands adiabatically and its volume becomes . The work done by the gas during isothermal and adiabatic expansion processes are and , then is _________ .
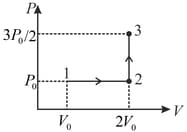
One mole of a monoatomic ideal gas goes through a thermodynamic cycle, as shown in the volume versus temperature diagram. The correct statement(s) is/are:
[ is the gas constant]
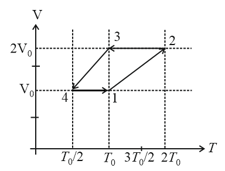
One mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where V is the volume and T is the temperature). Which of the statements below is (are) true?