Priyanka B Solutions for Chapter: Sample Paper, Exercise 1: Sample Paper 4
Priyanka B Chemistry Solutions for Exercise - Priyanka B Solutions for Chapter: Sample Paper, Exercise 1: Sample Paper 4
Attempt the practice questions on Chapter 6: Sample Paper, Exercise 1: Sample Paper 4 with hints and solutions to strengthen your understanding. Essentials of Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Priyanka B Solutions for Chapter: Sample Paper, Exercise 1: Sample Paper 4 with Hints & Solutions
A mixture containing two liquids visibly separated is placed in a separating funnel. Answer the following:
(i) What type of liquids form the mixture?
(ii) Which of the liquid will form lower layer?
(iii) What is the principle behind this method of separation?
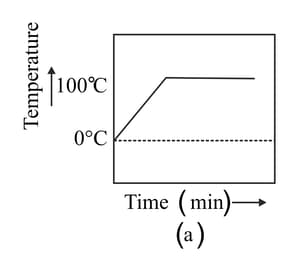
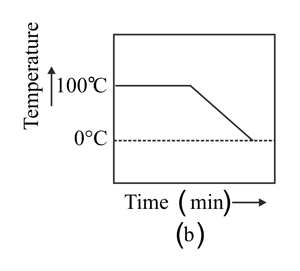
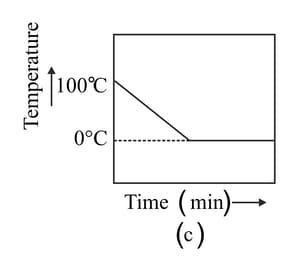
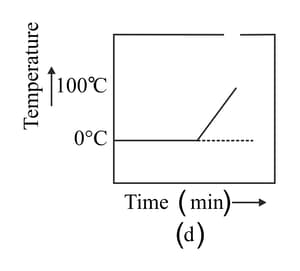
A student heats a beaker containing ice and water. He measures the temperature of the content of the beaker as a function of time. Which of the following figures would correctly represent the result? Justify your choice.
In the following table the mass number and the atomic number of certain elements are given:
| Element | A | B | C | D | E |
| Mass number | |||||
| Atomic number |
(A) State the pair of isobars from the above table.
(B) What would be the valency of the element C listed in the above table?
(C) Which two subatomic particles are equal in number in a neutral atom?
In a chemical reaction, the sum of the masses of the reactants and products remains unchanged. This is called the law of conservation of _____.
A group of atoms carrying a fixed charge on them is called _____.
The formula unit mass of is _____.
The following substances are added to water kept in a beaker:
(i) Sugar (ii) Milk (iii) Sand
Observe the mixtures created for stability (setting down of particles) and filterability.
How will you prepare (i) a mixture and (ii) a compound, using iron filings and sulphur. Can you just by seeing, judge that the mixture formed is a homogeneous and heterogeneous mixture?
