Embibe Experts Solutions for Exercise 4: Assignment
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Exercise 4: Assignment
Attempt the practice questions from Exercise 4: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 4: Assignment with Hints & Solutions
An ideal gas expands according to the law constant. The internal energy of the gas
Neon gas of a given mass expands isothermally to double volume. What should be the further fractional decrease in pressure, so that the gas when adiabatically compressed from that state, reaches the original state?
In a thermodynamic process two moles of a monatomic ideal gas obeys If temperature of the gas increases from to then find work done by the gas (where universal gas constant)
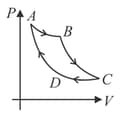
Carnot cycle is plotted in graph. Which portion represents an isothermal expansion?
Efficiency of a heat engine working between a given source and sink is . Coefficient of performance of the refrigerator working between the same source and the sink will be
A heat engine rejects to the sink at . Amount of work done by the engine will be (Temperature of source is
Entropy of a system decreases
Internal energy of an ideal gas depends on
