Maharashtra Board Solutions for Chapter: Inside the Atom, Exercise 14: Exercises
Maharashtra Board General Science Solutions for Exercise - Maharashtra Board Solutions for Chapter: Inside the Atom, Exercise 14: Exercises
Attempt the practice questions on Chapter 5: Inside the Atom, Exercise 14: Exercises with hints and solutions to strengthen your understanding. General Science solutions are prepared by Experienced Embibe Experts.
Questions from Maharashtra Board Solutions for Chapter: Inside the Atom, Exercise 14: Exercises with Hints & Solutions
Define moderator in nuclear reactor.
Fill in the blank with the correct word given in the bracket. (molecular particles/ sub-atomic particles)
Electrons, proton, neutron are the types of _____ in an atom.
An electron carries a _____charge.
The electron shell _____ is nearest to the nucleus.
The electronic configuration of magnesium is From this it is understood that the valence shell of Magnesium is_____.
The valency of hydrogen is 'one' as per the molecular formula . Therefore valency of 'Fe' turns out to be_____ as per the formula .
Match the pairs.
| Group A | Group B |
| a. Proton | i. Negatively charged |
| b. Electron | ii. Neutral |
| c. Neutron | iii. Positively charged |
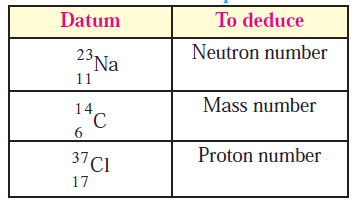
Deduce from the datum provided.