R P Rana Solutions for Chapter: Atomic Structure, Exercise 4: Exercise
R P Rana Science Solutions for Exercise - R P Rana Solutions for Chapter: Atomic Structure, Exercise 4: Exercise
Attempt the practice questions on Chapter 4: Atomic Structure, Exercise 4: Exercise with hints and solutions to strengthen your understanding. Its All About Science - Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from R P Rana Solutions for Chapter: Atomic Structure, Exercise 4: Exercise with Hints & Solutions
Draw the diagrams representing the atomic structure of the following:
Sodium atom (Atomic number 11; Mass No. 23)
Draw the diagrams representing the atomic structure of the following:
Chlorine atom (Atomic number 17; Mass No. 35).
Draw the diagram representing the atomic structure of the following:
Carbon atom (Atomic number 6; Mass No. 12).
What do you understand by isotopes? Give one example.
It is possible to write down the electronic configuration of an atom if we know its atomic number. Why this is so?
What would be the electronic configuration of a positively charged sodium ion? What would be its atomic number and mass number.
Which of the two would be chemically reactive, element X of atomic number 18 or element Z of atomic number 16? Explain your answer.
The electronic configuration of two elements X and Y are given below
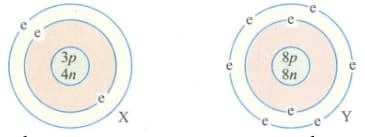
where p = proton, n = neutron, e = electron. When these two elements combine together to form a compound, give the mass (in grams) of one mole of this compound.
