Ramendra C Mukerjee Solutions for Chapter: Properties of Gases, Exercise 2: Objective Problems
Ramendra C Mukerjee Chemistry Solutions for Exercise - Ramendra C Mukerjee Solutions for Chapter: Properties of Gases, Exercise 2: Objective Problems
Attempt the practice questions on Chapter 12: Properties of Gases, Exercise 2: Objective Problems with hints and solutions to strengthen your understanding. Modern Approach to Chemical Calculations solutions are prepared by Experienced Embibe Experts.
Questions from Ramendra C Mukerjee Solutions for Chapter: Properties of Gases, Exercise 2: Objective Problems with Hints & Solutions
If the product of the gas constant i.e., and temperature in kelvin equals the compressibility factor of the gas at atmospheric pressure is
The graph of the quantity against pressure is extrapolated to zero pressure to obtain a limiting value. If, this limiting value for a certain non-ideal gas is found to be at , the molar mass of the gas will be:
The root mean square speed of molecules in a gas sample can be changed by:
How high must a column of water be to exert a pressure equal to that of a column of , that is high
In the following graph in which volume is plotted versus temperature, the lines and , represent the same mass of the same ideal gas at different pressures and , respectively.
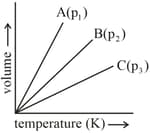
The correct relationship of pressures is
A reaction vessel contain hydrogen at a partial pressure of and oxygen gas at a partial pressure of . The limiting reactant in the following reaction: is:
When a gas is passed through a small hole at a temperature greater than its critical temperature, Joule-Thomson effect will show:
Which of the following statements is wrong?
