NCERT Solutions for Chapter: Equilibrium, Exercise 1: Matching Type
NCERT Chemistry Solutions for Exercise - NCERT Solutions for Chapter: Equilibrium, Exercise 1: Matching Type
Attempt the practice questions on Chapter 7: Equilibrium, Exercise 1: Matching Type with hints and solutions to strengthen your understanding. NCERT Exemplar Chemistry - Class 11 solutions are prepared by Experienced Embibe Experts.
Questions from NCERT Solutions for Chapter: Equilibrium, Exercise 1: Matching Type with Hints & Solutions
Match the following equilibria with the corresponding condition
| (i) | LiquidVapour | (a) | Saturated solution |
| (ii) | SolidLiquid | (b) | Boiling point |
| (iii) | Solid Vapour | (c) | Sublimation point |
| (iv) | Solute (s)Solute (solution) | (d) | Melting point |
| (e) | Unsaturated solution |
For the reaction :
Equilibrium constant
Some reactions are written below in Column I and their equilibrium constants in terms of are written in Column II. Match the following reactions with the corresponding equilibrium constant.
| Column I (Reaction) | Column II (Equilibrium constant) | ||
| (i) | (a) | ||
| (ii) | (b) | ||
| (iii) | (c) | ||
| (d) | |||
Match standard free energy of the reaction with the corresponding equilibrium constant
| Column I | Column II | ||
| (i) | (a) | ||
| (ii) | (b) | ||
| (iii) | (c) | ||
| (d) |
Match the following species with the corresponding conjugate acid
| Species | Conjugate acid | ||
|
(i) |
(p) | ||
| (ii) | (q) | ||
| (iii) | (r) | ||
| (iv) | (s) | ||
| (t) | |||
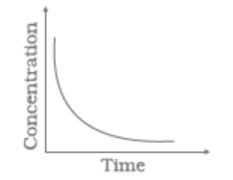
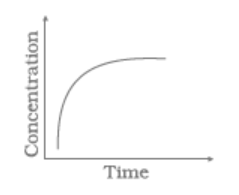
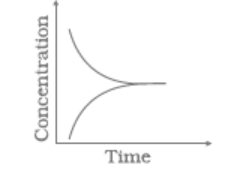
Match the following graphical variation with their description
| A | B | ||
| (i) |
|
(a) | Variation in product concentration with time |
| (ii) |
|
(b) | Reaction at equilibrium |
| (iii) |
|
(c) | Variation in reactant concentration with time |
Match Column (I) with Column (II).
| Column I | Column II | ||
| (i) | Equilibrium | (a) | |
| (ii) | Spontaneous reaction | (b) | |
| (iii) | Non-spontaneous reaction | (c) | |
| (d) | |||