Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 2: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 2: EXERCISES
Attempt the free practice questions on Chapter 2: Acids, Bases and Salts, Exercise 2: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 10 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Acids, Bases and Salts, Exercise 2: EXERCISES with Hints & Solutions
Ayaan tested the nature of a few common substances with phenolphthalein indicator and summarised the results in the following table.
| Test tubes | Colour change with phenolphthalein indicator |
| 1 | Colourless |
| 2 | Pink |
| 3 | Colourless |
| 4 | Pink |
Which of the following substances could be present in test tubes and ?
| 1 | 2 | 3 | 4 | |
| (A) | Lime juice | Soda water | Lime Water | Vinegar |
| (B) | Common salt | Lemon juice | Vinegar | Lime Water |
| (C) | Lemon juice | Lime water | Common salt solution | Baking soda |
| (D) | Baking soda | Lemon juice | Lime Water | Soda water |
While demonstrating acid base reactions, MS. Prabha, a science teacher added sodium oxide to until it was in excess. Which of the following graphs correctly represents the change in ?
Aqueous solutions of salts are either acidic, basic or neutral. A few common salts are listed as:
I. Silver chloride
II. Ammonium sulphate
III. Sodium nitrate
IV. Sodium phosphate
V. Sodium acetate
Which of the following correctly matches the given salts with the nature of their aqueous solutions?
Brine solution
| Solution with | Solution with | Solution with | |
| (A) | I,III | II,IV | V |
| (B) | I,II | IV,V | III |
| (C) | I | IV,II | III,V |
| (D) | II | I,IV,III | V |
Study the given flow chart carefully and fill in the blanks by choosing an appropriate option.
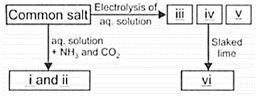
| I | II | III | IV | V | VI | |
| (A) | ||||||
| (B) | ||||||
| (C) | ||||||
| (D) |
Observe the given figure carefully, which represents the decomposition of brine solution by passing electricity.
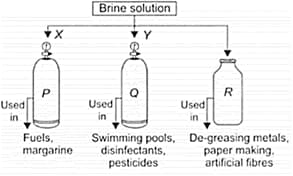
| X | Y | P | Q | R | |
| (A) | At anode | At Cathode | gas | gas | |
| (B) | At anode | At Cathode | gas | gas | |
| (C) | At anode | At Cathode | gas | gas | |
| (D) | At anode | At Cathode | gas | gas |
