Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 2: EXERCISES
Anil Ahlawat Science Solutions for Exercise - Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 2: EXERCISES
Attempt the free practice questions on Chapter 3: Metals and Non-Metals, Exercise 2: EXERCISES with hints and solutions to strengthen your understanding. NSO Science Olympiad Workbook Grade 10 solutions are prepared by Experienced Embibe Experts.
Questions from Anil Ahlawat Solutions for Chapter: Metals and Non-Metals, Exercise 2: EXERCISES with Hints & Solutions
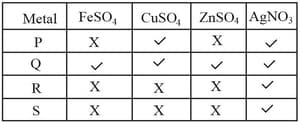
Ritika took four metals and added them to the following solutions one by one. The results are summarised in the given table.
- Among the given metals, is the most reactive metal. Which of the following statements are correct?
- is less reactive than but more reactive than .
- is less reactive than but more reactive than .
- is more reactive than but less reactive than .
Observe the given figure carefully.
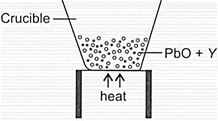
The residue left behind in the crucible, substance and the substance which can replace in the above process are respectively
Read the given statements carefully.
- Metals are generally ductile and metal is the most ductile metal.
- Metals generally possess high melting points but few metals like have low melting point.
- Metals are good conductors of electricity but metal is very poor conductor of electricity.
- Among metals, is the poorest conductor of heat.
Read the given passage carefully and fill in the blanks by selecting an appropriate option.
Oxides of are reduced to their respective metals by heating with coke and the process is called . Before carrying out reduction with carbon, if the calcined or roasted ore still contains infusible impurities of earthy matter, . an additional substance, is added which combines with iii to form a fusible material, called .
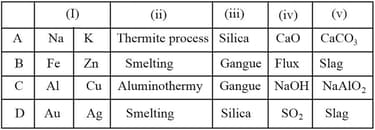
Read the given statements carefully and state for true and for false ones.
I. Metals of low reactivity can be extracted directly by the electrolysis of their molten ores.
II. Prior to reduction, metal sulphides and carbonates must be converted to metal oxides.
III. Metals of high reactivity can be extracted by electrolytic oxidation.
IV. Thermite reaction is a displacement reaction and is highly exothermic.
| I | II | III | IV | |
|---|---|---|---|---|
| (A) | ||||
| (B) | ||||
| (C) | ||||
| (D) |