B M Sharma Solutions for Chapter: Thermodynamics, Exercise 1: ILLUSTRATIONS
B M Sharma Physics Solutions for Exercise - B M Sharma Solutions for Chapter: Thermodynamics, Exercise 1: ILLUSTRATIONS
Attempt the practice questions on Chapter 4: Thermodynamics, Exercise 1: ILLUSTRATIONS with hints and solutions to strengthen your understanding. PHYSICS FOR JOINT ENTRANCE EXAMINATION WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from B M Sharma Solutions for Chapter: Thermodynamics, Exercise 1: ILLUSTRATIONS with Hints & Solutions
An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
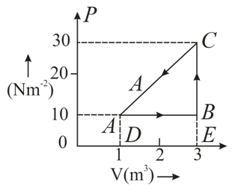
What is the internal energy of the gas at point
An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
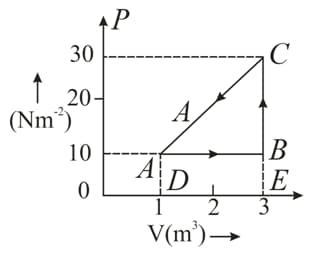
How much heat energy is absorbed by the gas in the process
An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
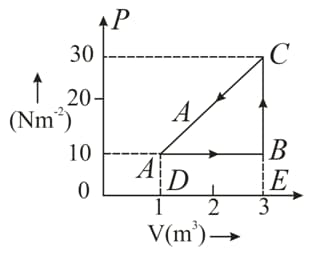
Find the heat energy rejected or absorbed by the gas in the process .
(d) What is the net work done by the gas in the complete cycle $A B C A ?$
An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
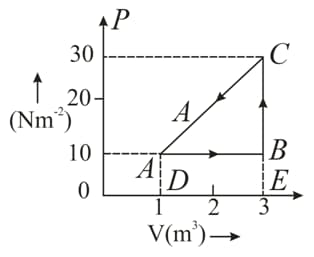
What is the net work done by the gas in the complete cycle
