D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 2: INTRODUCTORY EXERCISE 21.2
D. C. Pandey Physics Solutions for Exercise - D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 2: INTRODUCTORY EXERCISE 21.2
Attempt the practice questions on Chapter 5: Laws of Thermodynamics, Exercise 2: INTRODUCTORY EXERCISE 21.2 with hints and solutions to strengthen your understanding. Understanding Physics JEE Main & Advanced WAVES AND THERMODYNAMICS solutions are prepared by Experienced Embibe Experts.
Questions from D. C. Pandey Solutions for Chapter: Laws of Thermodynamics, Exercise 2: INTRODUCTORY EXERCISE 21.2 with Hints & Solutions
How many moles of Helium at temperature and pressure are needed to make the internal energy of the gas ?
Temperature of four moles of a monoatomic gas is increased by in isochoric process. Find and .
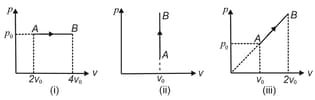
Find the work done by the gas in the process shown in the following figures:
Temperature of two moles of an ideal gas is increased by in a process, where, is a positive constant. Find the work done by the gas in the given process.
The pressure and the volume of a gas changes from, to in a process, constant. Find the work done by the gas in the given process.
One mole of an ideal monoatomic gas is initially at . Find the final temperature if of heat is added,
(a) at constant volume.
(b) at constant pressure.
An ideal gas expands while the pressure is kept constant. During this process, does the heat flow into or out of the gas? Justify your answer.