In a , a homogeneous reaction's first-order of the type (consecutive reaction) is carried out. Among the following, which curve shows a variation of concentration of and with time?
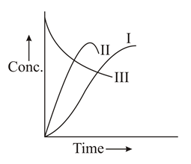
In a , a homogeneous reaction's first-order of the type (consecutive reaction) is carried out. Among the following, which curve shows a variation of concentration of and with time?


Important Questions on Chemical Kinetics
A substance undergoes a first-order decomposition. The decomposition follows two parallel first-order reactions as given below.
The percentage distributions of and are
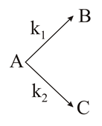
( and )
Consider the elementary reaction sequence shown in the figure. Among the following equations, which one is correct?
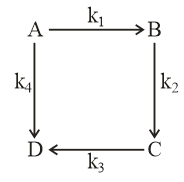
,
where and are the volume of standard needed to neutralize acid present at a given time. If ester is neutralised then
Inversion of sucrose is the first-order reaction and is studied by measuring the angle of rotation at different intervals of time
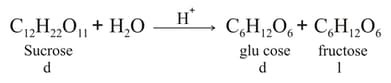 .
.
If and (where, and are the angles of rotation at the start, at the time and at the end of the reaction, respectively), then there is inversion when:
Consider the decay of to and by two parallel first-order reactions as shown in the figure
| Reaction | Rate constant | Energy of activation | |
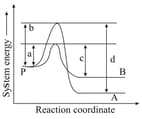
Which of the following is (are) true?
