MEDIUM
10th ICSE
IMPORTANT
Earn 100
of kerosene oil at is mixed with of water at . What is the temperature of the mixture? Specific heat of kerosene .

Important Questions on Calorimetry
HARD
10th ICSE
IMPORTANT
HARD
10th ICSE
IMPORTANT
MEDIUM
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
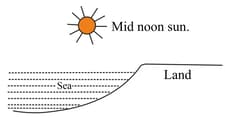
The figure given below shows a geographical phenomenon. Name this phenomenon and explain it in brief.
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
