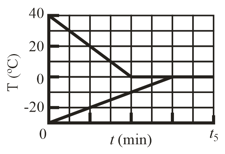
A sample of liquid water and a sample of ice are placed in a thermally insulated container. The container also contains a device that transfers energy as heat from the liquid water to the ice at a constant rate until thermal equilibrium is reached. The temperatures of the liquid water and the ice are given in the figure below as functions of time ; the horizontal scale is set by
(a) What is rate
(b) What is the initial mass of the ice in the container?
(c) When thermal equilibrium is reached, what is the mass of the ice produced in this process?
(a) What is rate
(b) What is the initial mass of the ice in the container?
(c) When thermal equilibrium is reached, what is the mass of the ice produced in this process?


Important Questions on Temperature, Heat, and the First Law of Thermodynamics
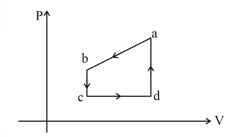
Given figure represents a closed cycle for gas (the figure is not drawn to scale). The change in the internal energy of the gas as it moves from to along the path is . As it moves from to must be transferred to it as heat. An additional transfer of to it as heat is needed as it moves from to . How much work is done on the gas as it moves from to ?
In the figure (a) two identical rectangular rods of metal are welded end to end, with a temperature of on the left side and a temperature of on the right side. In is conducted at a constant rate from the right side to the left side. How much time would be required to conduct if the rods were welded side to side as in Figure (b)?