HARD
JEE Advanced
IMPORTANT
Earn 100
A Carnot engine takes of heat from a reservoir at and gives it to a sink at . Find the work done by the engine.

Important Questions on Laws of Thermodynamics
HARD
JEE Advanced
IMPORTANT
The efficiency of a carnot cycle is . If on reducing the temperature of the sink by, the efficiency becomes , find the source and the sink temperatures between which the cycle is working.
HARD
JEE Advanced
IMPORTANT
Refrigerator works between and while refrigerator works between and , both removing heat equal to from the freezer. Which of the two is a better refrigerator?
HARD
JEE Advanced
IMPORTANT
A refrigerator has to transfer an average of heat per second from a temperature of to . Calculate the average power consumed assuming that no energy is lost in the process.
HARD
JEE Advanced
IMPORTANT
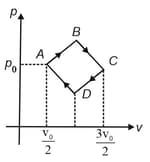
moles of a monoatomic gas is taken around in a cyclic process consisting of four processes along as shown. All the lines on the diagram have a slope of magnitude . The pressure at and is and the volumes at and are and , respectively. Calculate the percentage efficiency of the cycle.
MEDIUM
JEE Advanced
IMPORTANT
In a process, the pressure of an ideal gas is proportional to square of the volume of the gas. If the temperature of the gas increases in this process, what is the work done by this gas?
HARD
JEE Advanced
IMPORTANT
moles of a gas are filled in a container at temperature . If the gas is slowly and isothermally compressed to half its initial volume, then the work done by the atmosphere on the gas is,

MEDIUM
JEE Advanced
IMPORTANT
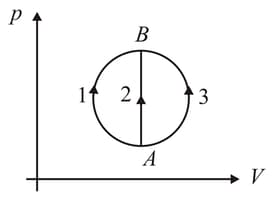
A gas undergoes to , through three different processes and , as shown in the figure. The heat supplied to the gas is and , respectively. Then,
HARD
JEE Advanced
IMPORTANT
An ideal gas has initial volume and pressure. In doubling its volume, the minimum work done will be in which of the process (of the given processes)?
