EASY
10th ICSE
IMPORTANT
Earn 100
A calorimeter of heat capacity contains of liquid at . A piece of metal of mass is heated up to and put in the calorimeter. The final temperature of mixture is obtained . If specific heat of metal is , calculate the specific heat of the liquid.

Important Questions on Calorimetry
EASY
10th ICSE
IMPORTANT
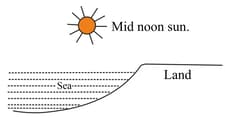
The figure given below shows a geographical phenomenon. Name this phenomenon and explain it in brief.
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
EASY
10th ICSE
IMPORTANT
