EASY
NEET
IMPORTANT
Earn 100
A carnot engine is made to work between and first and, then, between and . The ratio of the efficiencies of the engine in the two cases is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
An ideal gas heat engine operates in a carnot cycle between and . It absorbs at the higher temperature. The amount of heat (in ) converted into work is equal to
EASY
NEET
IMPORTANT
Efficiency of a carnot engine is when temperature of outlet is . In order to increase its efficiency up to , keeping temperature of intake the same, what should be the temperature of outlet?
MEDIUM
NEET
IMPORTANT
The diagram below represents the thermodynamic cycle of an engine operating with an ideal monatomic gas. The amount of heat extracted from the source in a single cycle and the efficiency of the cycle are, respectively,
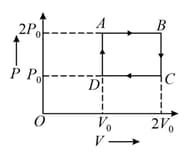
MEDIUM
NEET
IMPORTANT
A carnot engine whose efficiency is takes in heat from a source, maintained at a temperature of . It is desired to have an engine of efficiency . Then the intake temperature for the same exhaust (sink) temperature must be
