A certain organic compound A decomposes by two parallel first order mechanism
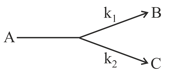
If and
Calculate the concentration ratio of to , if experiment is started with only and allowed to run for one hour.

Important Questions on Chemical Kinetics
 Suppose the half life values for the two branches are minutes and minutes, what is the overall half-life value?
Suppose the half life values for the two branches are minutes and minutes, what is the overall half-life value?The catalytic decomposition of formic acid may take place in two ways :
(a)
(b)
The rate constant and activation energy for reaction (a) are at and kcal . respectively and for reaction (b) are at and respectively. Find the temperature which will give a product made up of equimolar quantities of water vapour, carbon monoxide, hydrogen and carbon dioxide.
For the following first order gaseous reaction
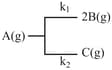
The initial pressure in a container of capacity litres is . Pressure at time is and after infinite time it becomes atmosphere. Find the rate constant and for the appropriate reactions.
