A certain reactant is getting converted to in solution. The rate constant of this reaction is measured by titrating a volume of the solution with a reducing reagent which only reacts with and . In this process, it converts to and to . At , the volume of the reagent consumed is and at , the volume used up is . Calculate the rate constant of the conversion of to assuming it to be a first order reaction.

Important Questions on Chemical Kinetics
The catalytic decomposition of formic acid may take place in two ways:
(a)
(b)
The rate constant and activation energy for reaction (a) are at and respectively and for reaction (b) are at and respectively. Find the temperature which will give a product made up of equimolar quantities of water vapour, carbon monoxide, hydrogen and carbon dioxide.
For the following first order gaseous reaction
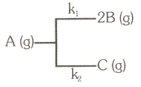
The initial pressure in a container of capacity litters is . Pressure at time is and after infinite time it becomes atmosphere. Find the rate constant and for the appropriate reactions.
Isomerization of cyclobutene into 1,3 -butadine follow first order kinetics as :
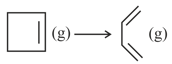
The kinetic study was performed by taking same amounts of cyclobutene in three sealed flasks. First flask was broken after 20 minute and the reaction mixture was absorbed completely in bromine solution. bromine solution was required. The second flask was broken after a very long time and the reaction mixture required bromine solution of the same strength. If the third flask was broken after 30 minute, what volume of bromine solution of same strength would have been required?
Decomposition of both and Follows 1st order kinetic as :
If one mole of each and Are taken in a Evacuated flask and heated to some temperature so that they start decomposing at the same rate, determine total pressure in the flask after .
At room temperature orange juice gets spoilt in about 64 hours. In a refrigerator at juice can be stored three times as long before it gets spoilt. Estimate
(a) the activation energy of the reaction that causes the spoiling of juice.
