MEDIUM
JEE Main
IMPORTANT
Earn 100
A closed hollow insulated cylinder is filled with gas at and also contains an insulated piston of negligible weight and negligible thickness at the middle point. The gas on one side of the piston is heated to . If the piston moves , the length of the hollow cylinder is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory Of Gases
HARD
JEE Main
IMPORTANT
The airtight and smooth pistons of a cylindrical vessel are connected with a string, as shown, initially, pressure and temperature of the gas are and . The atmospheric pressure is also . At a later time, the tension in the string is where is the cross-sectional area of the cylinder. At this time, the temperature of the gas has become

MEDIUM
JEE Main
IMPORTANT
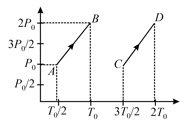
graph for the same number of moles of two ideal gases is shown in the figure. Find the path along which volume decreases.
MEDIUM
JEE Main
IMPORTANT
A vessel of volume is separated into three equal parts by stationary semipermeable membranes. The left, middle and right parts are filled with of hydrogen, of oxygen and of nitrogen respectively. The left partition lets through the only hydrogen while the right partition lets through hydrogen and nitrogen. If the temperature in all is , the ratio of pressure in the three compartments will be?
MEDIUM
JEE Main
IMPORTANT
A steel tank contains of ammonia gas at a pressure of and a temperature of . Later the temperature is and the pressure is .
HARD
JEE Main
IMPORTANT
Which of the following quantities is independent of the nature of the gas at same temperature?
HARD
JEE Main
IMPORTANT
Which of the following quantities depend on temperature only for a given ideal gas?
