A common window is to be replaced with a better insulated one. The initial window has glass with thickness . The new window has two such glass layers, separated by , with the intermediate space containing air. For
(a) the common window and
(b) the replacement.
Find the energy loss in watts per square meter when the outside temperature is and the inside temperature is . (Given for glass thermal conductivity )

Important Questions on Temperature, Heat, and the First Law of Thermodynamics
One way to keep the contents of the garage from becoming too cold at night when there is a severe subfreezing temperature in the forecast is to put a tube of water inside the garage. If the mass of the water is and its initial temperature is ,
(a) How much energy must the water transfer to its surrounding in order to freeze completely? and
(b) What is the lowest possible temperature of the water and its surrounding until that happens?
Non-metric version:
(a) How long does a water heater take to raise the temperature of gal of water from to ?
Metric version:
(b) How long does a water heater take to raise the temperature of of water from to ? [Take specific heat of water , for water at , ]
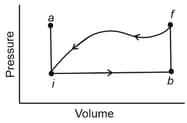
A lab sample of gas is taken through cycle shown in the diagram, net work done is . Along path , the change in the internal energy is and the magnitude of the work done is . Along path the energy transferred to the gas as heat is . How much energy is transferred as heat along
(a) path and
(b) path ?
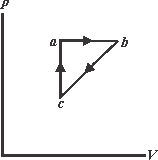
When a system is taken from state to state along path . In the figure and . Along the path .
(a) What is along the path ?
(b) If for the return path , what is the for this path?
(c) If . What is ?
If , what is for,
(d) path and
(e) path ?
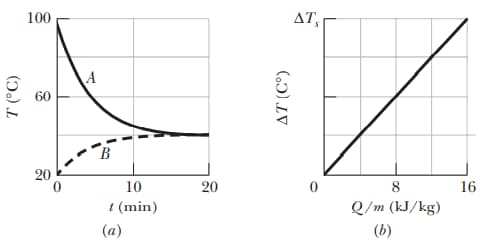
Samples and are at different initial temperatures when they are placed in a thermally insulated container and allowed to come to thermal equilibrium. Figure gives their temperatures versus time . Sample has a mass of sample has a mass of . Figure is a general plot for the material of sample . It shows the temperature change that the material undergoes when energy is transferred to it as heat . The change is plotted versus the energy per unit mass of the material, and the scale of the vertical axis is set by . What is the specific heat of sample ?
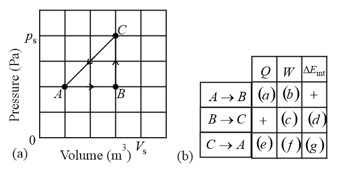
A thermodynamic system is taken from state to state to state and then back to , as shown in the diagram of figure (a). The vertical scale is set by , and the horizontal scale is set by (a) Complete the table in figure (b) by inserting a plus sign, a minus sign, or a zero in each indicated cell. (h) What is the net work done by the system as it moves once through the cycle
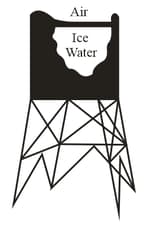
A slab has formed on an outdoor tank of water (as shown in Fig.). The air is at . Find the rate of ice formation (). The ice has thermal conductivity and density . Assume there is no energy transfer through the walls or bottom.