EASY
NEET
IMPORTANT
Earn 100
A compound exists in the gaseous phase, both as a monomer and a dimer . The atomic mass of is , and the molecular mass of is . In an experiment, of the compound was confined in a vessel of volume litres, and heated to . The pressure developed, if the compound exists as dimer to the extent of by weight under these conditions, will be
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
See the figure-:
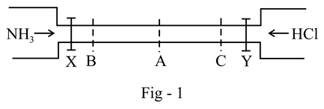
The valves of and are opened simultaneously. The white fumes of will first form at:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
