A diatomic gas $(\gamma=1.4)$ does work when it is expanded isobarically. The heat given to the gas in this process is,

Important Questions on Kinetic Theory of Gases and Radiations
A thermodynamic system is taken through the cycle PQRSP process. The net work done by the system is,
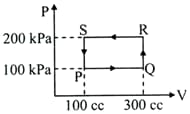
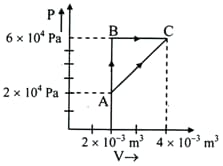
Figure below shows two paths that may be taken by a gas to go from a state $A$ to a state $C$. In process $A B, 400\; \mathrm{J}$ of heat is added to the system and in process ${BC}, 100 \;\mathrm{J}$ of heat is added to the system. The heat absorbed by the system in the process will be,
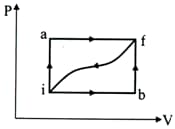
When a system is taken from state $i$ to a state $f$ along path $iaf$, $Q=50 \;\mathrm{J}$ and ${W}=20 \;\mathrm{J}$. Along path $ibf$, $Q=35\;\mathrm{J}$ . If $W=-13\;\mathrm{J}$ for the curved return path $\mathrm{fi}, {Q}$ for this path is
One mole of an ideal gas goes from an initial state $A$ to final state $B$ via two processes: it first undergoes isothermal expansion from volume $V$ to $3{V}$ and then its volume is reduced from $3{V}$ to ${V}$ at constant pressure. The correct diagram representing the two processes is,
Which of the following statement is true?
Absorption coefficient of totally black body is
If coefficient of absorption of a body is and coefficient of reflection is , then a value of coefficient of transmission is
