MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
A diatomic molecule is made of two masses and which are separated by a distance . If we calculate its rotational energy by applying Bohr’s rule of angular momentum quantization, its energy will be given by : ( is an integer)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atomic Physics
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
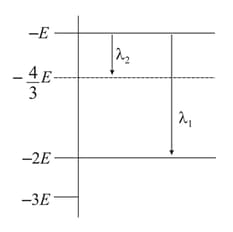
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths is given by :
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
